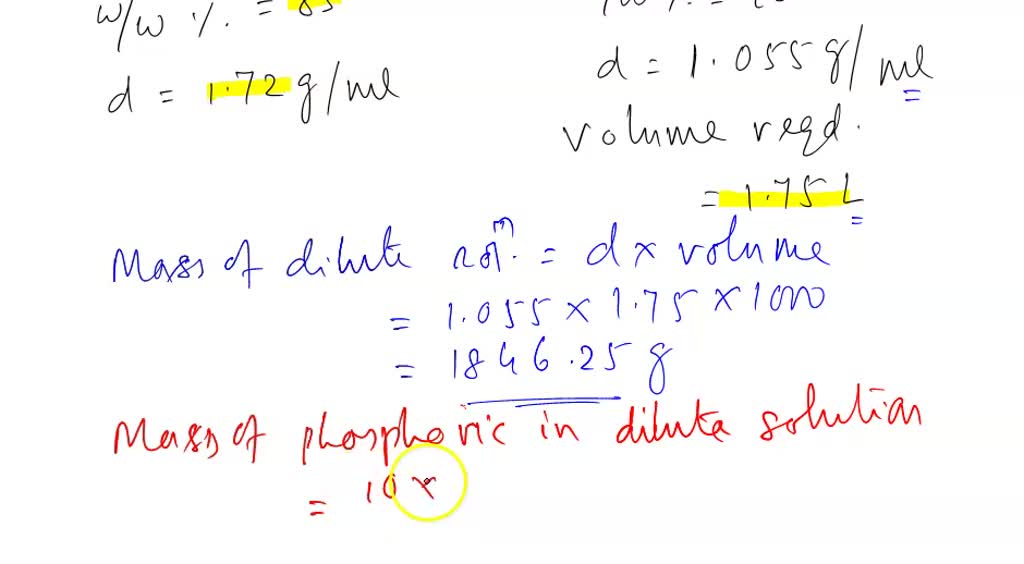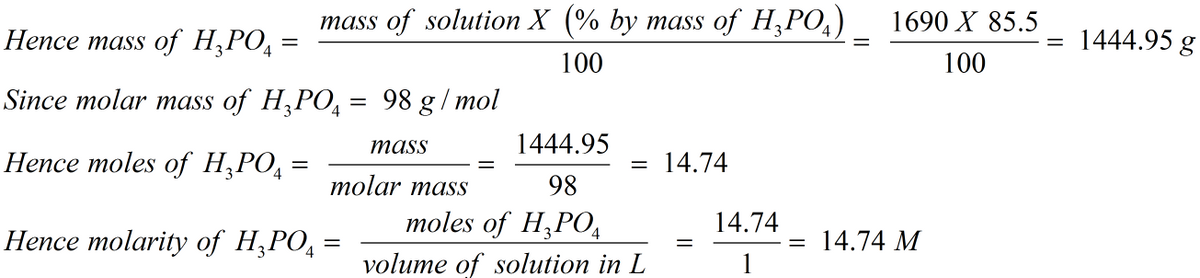✓ Solved: A solution of phosphoric acid, H3 PO4, is found to contain 35.2 g of H3 PO4 per liter of solution....

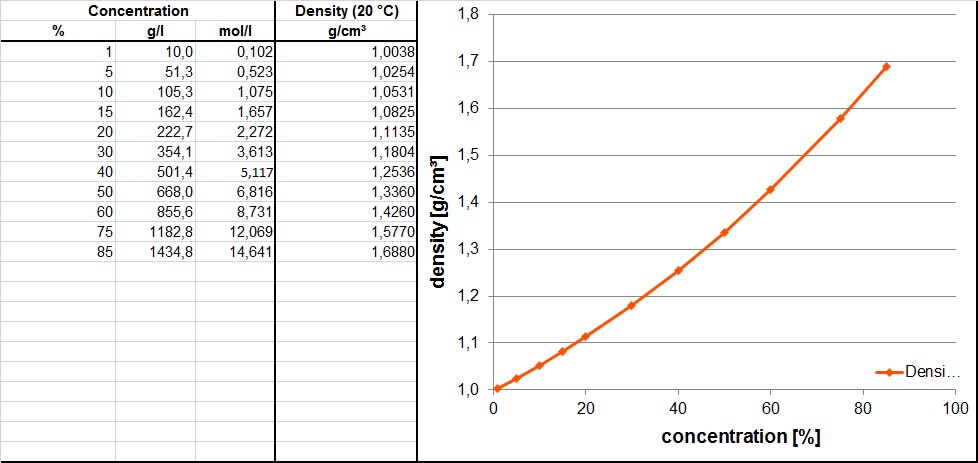
The density of 85% Phosphoric acid is 1.70 g cm^-3 . What is the volume of a solution that contains 17 gm Phosphoric acid?

A concentrated phosphoric acid solution is 85.doc - A concentrated phosphoric acid solution is 85.5% H3PO4 by mass and has a density of 1.69 g/mL at | Course Hero
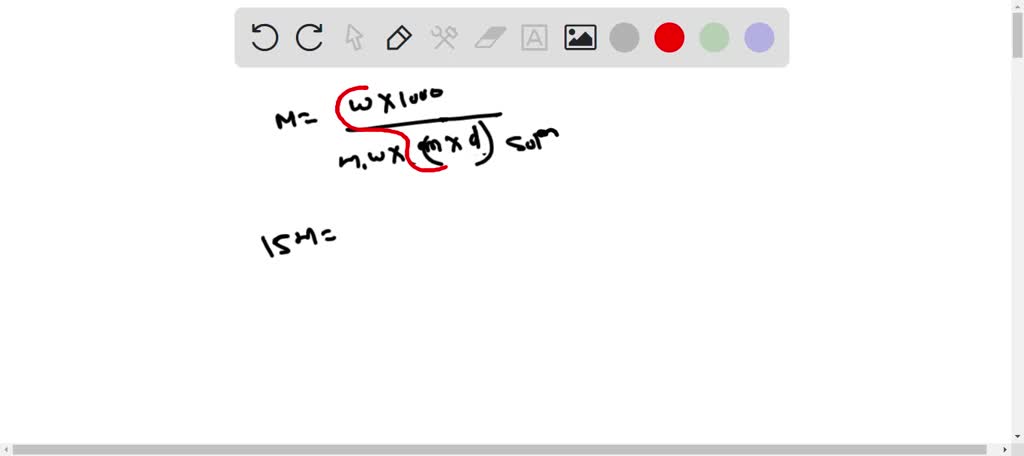
How will you prepare 100ml of 6m orthophosphoric acid mol weight =98; specific gravity=1.75minimum assay-85%? - Quora
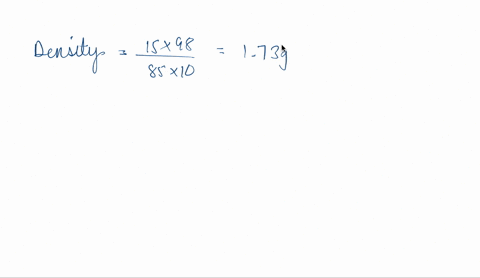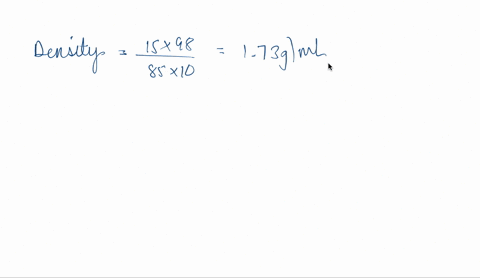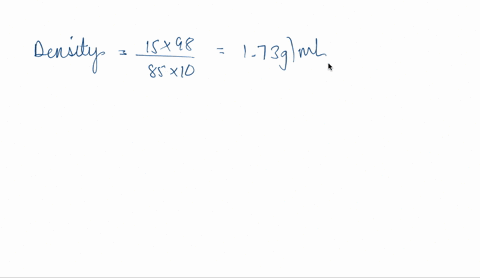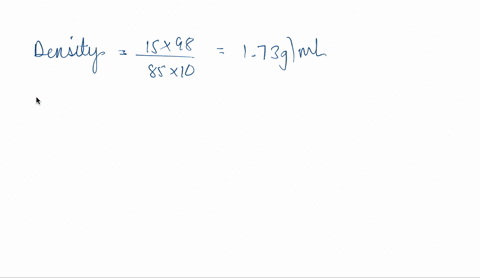
SOLVED: phosphoric acid, 85% purity density =1.88 g/ml) AND the Calculate the volume of commercial of water needed to prepare SOOmL of 0.5 M phosphoric acid solution. (MW: 98g/mol) volume (1 pt)
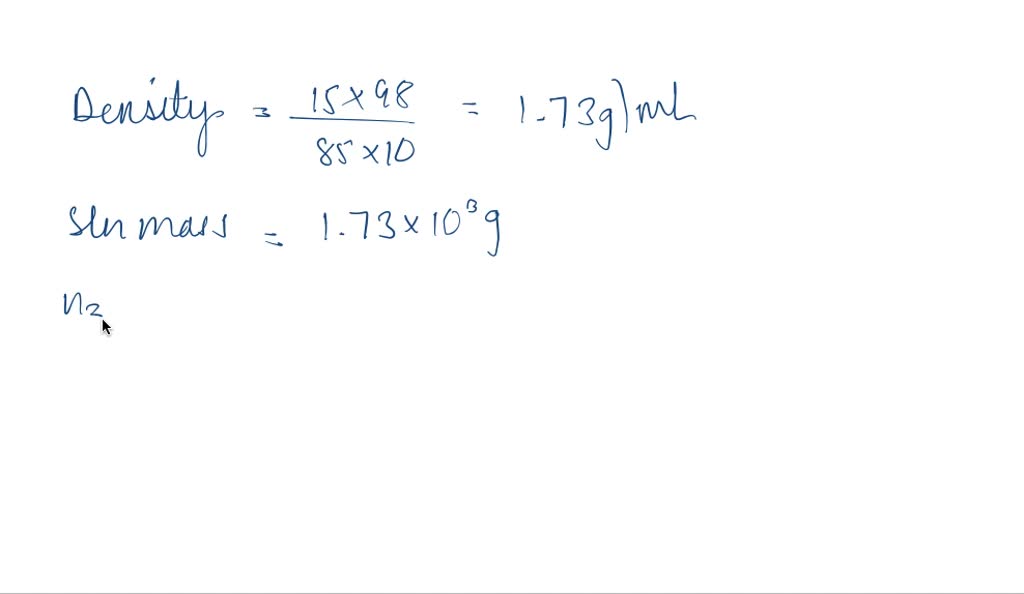
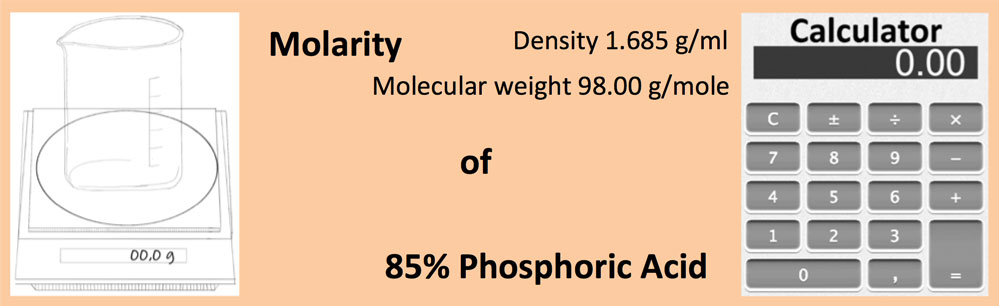
SOLVED: Q94. What is the molarity of a phosphoric acid solution if the solution is 85% by mass H3PO4 and has a density of 1.7 g/mL? [Hint: the solute is H3PO4 and

SOLVED: A concentrated phosphoric acid solution is 85.5% H3PO4 by mass and has a density of 1.69 g/mL at 25°C. What is the molarity of H3PO4? What is the mole fraction of
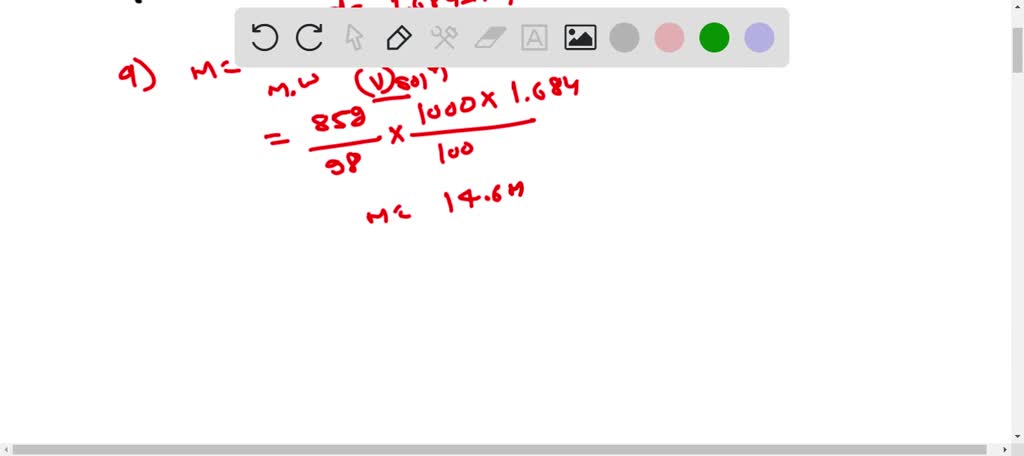
SOLVED: A solution of concentrated phosphoric acid contains 85.0% H3PO4 by mass and has a density of 1.684 g/mL. (1 mol H3PO4 = 98.03 g, 1 mol H2O = 18.02 g, and
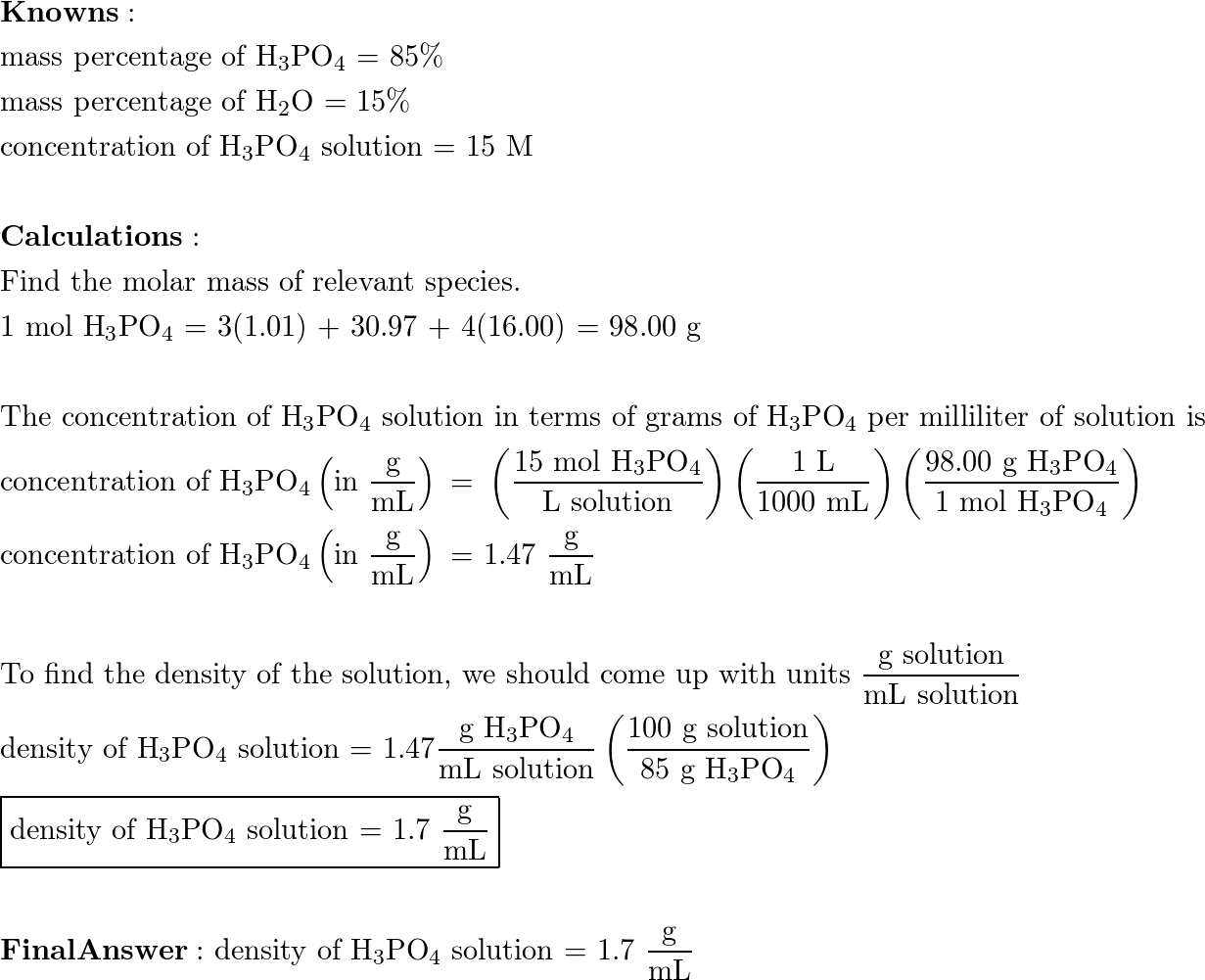
SOLVED: Concentrated phosphoric acid is sold as a solution of 85 % phosphoric acid and 15 % water by mass. Given that its molarity is 15 M, calculate the density of concentrated phosphoric acid.