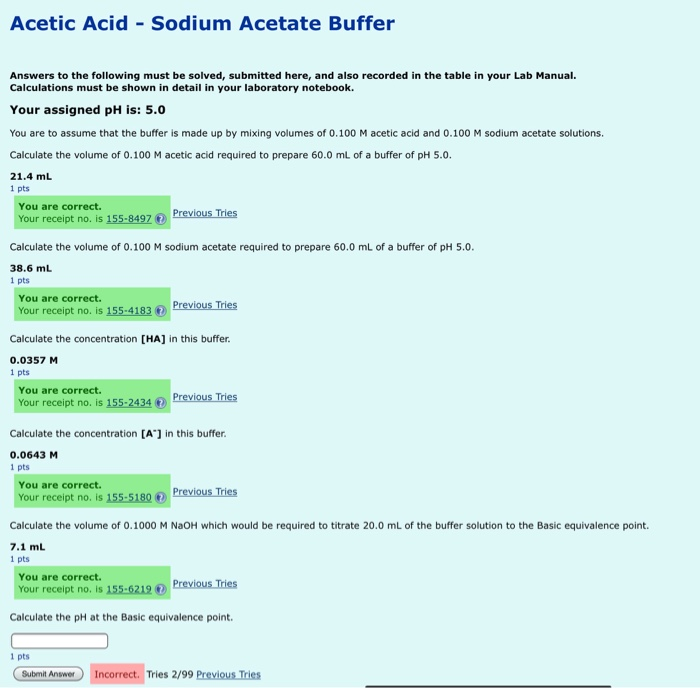
![SOLVED: [10]4. The pH of a sodium acetate-acetic acid buffer is 4.5. Calculate the ratio of [CH3COOH]/[CH3COO-]. [Ka of CH3COOH is 1.8x10-5] SOLVED: [10]4. The pH of a sodium acetate-acetic acid buffer is 4.5. Calculate the ratio of [CH3COOH]/[CH3COO-]. [Ka of CH3COOH is 1.8x10-5]](https://cdn.numerade.com/ask_previews/03ad68ba-cab0-4fc6-ad7e-62059a06cfe3_large.jpg)
SOLVED: [10]4. The pH of a sodium acetate-acetic acid buffer is 4.5. Calculate the ratio of [CH3COOH]/[CH3COO-]. [Ka of CH3COOH is 1.8x10-5]
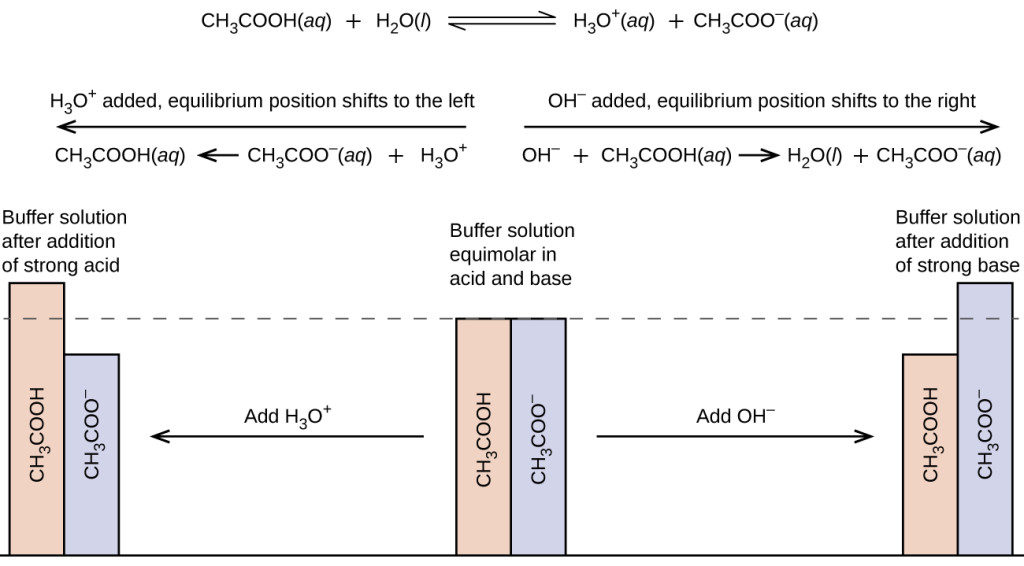
Compare 1 L of acetate buffer solution (0.50 mol of acetic acid and 0.50 mol sodium acetate) to 1 L of HCl solution Similarities
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/dDlCNVZnUE9URzQ=/sd/)
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]

You have 250mL of a 0.56M solution of sodium acetate. How many mL of 0.50M acetic acid should be added to make a buffer of pH 4.40? | Homework.Study.com

SOLVED: Problem Facing: You have to prepare a 3 Liter of 0.2 M Acetate Buffer, pH 5.0, using sodium acetate trihydrate (MW 136) and M solution of Acetic Acid. Calculate the amount

SOLVED:A biochemist needs 750 mL of an acetic acid-sodium acetate buffer with pH 4.50. Solid sodium acetate (CH3 COONa) and glacial acetic acid (CH3 COOH) are available. Glacial acetic acid is 99 %
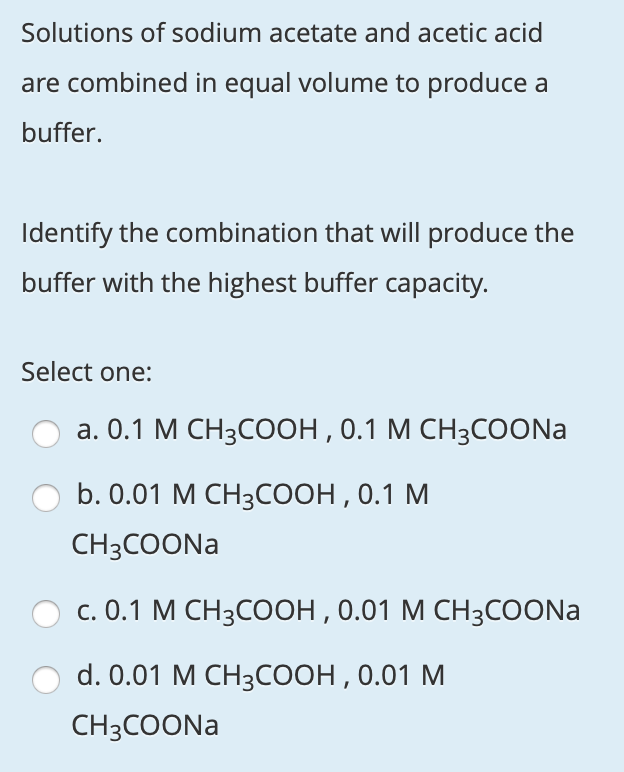
For preparing a buffer of pH 6 by mixing sodium acetate and acetic acid the ratio of the concentration of salt and acid should be ( Ka=10^ 5)

A buffer solution is prepared by mixing `10ml` of `1.0 M` acetic acid & `20 ml` of `0.5 M` - YouTube

BUFFERS Mixture of an acid and its conjugate base. Buffer solution resists change in pH when acids or bases are added or when dilution occurs. Mix: A. - ppt download