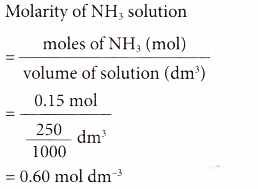
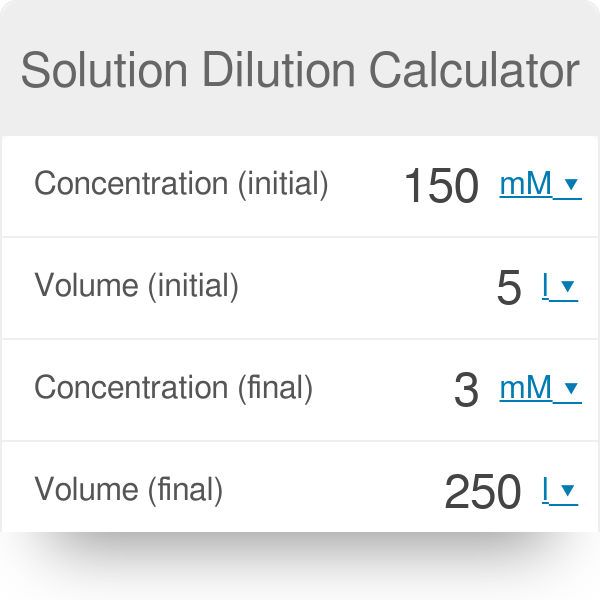
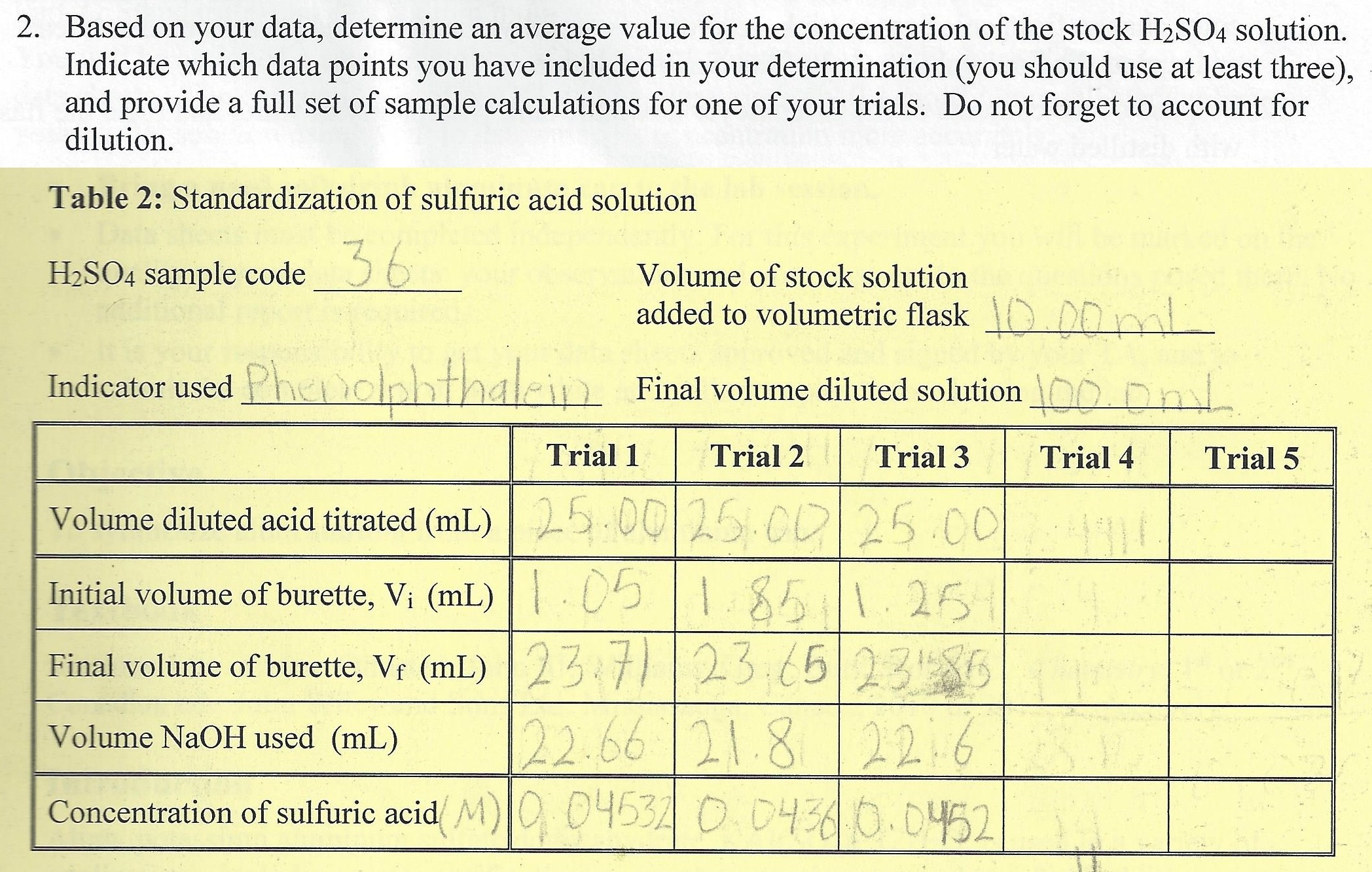
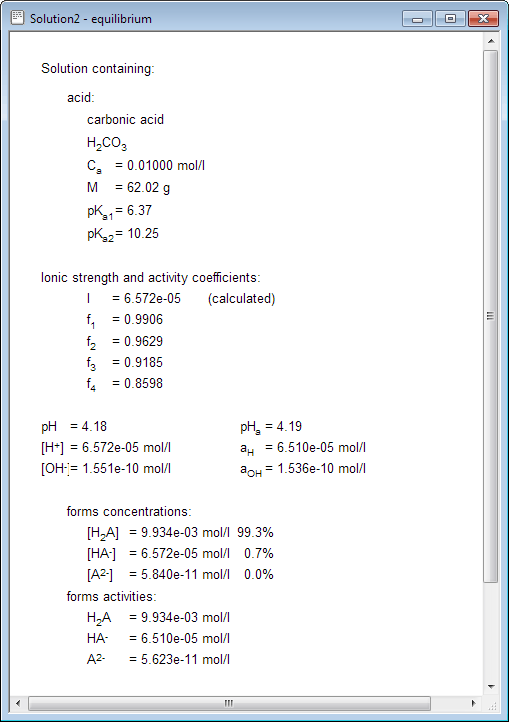
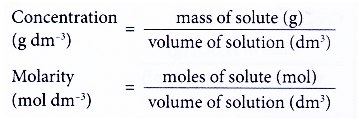How to calculate the concentrations of conjugate species present in the solution of diprotic acid? - Chemistry Stack Exchange
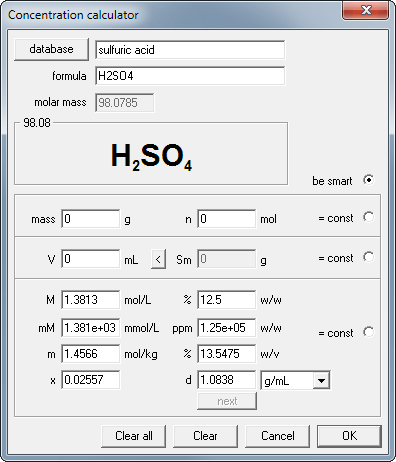
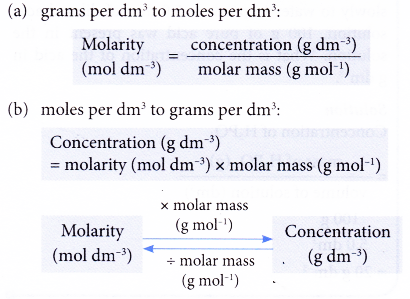
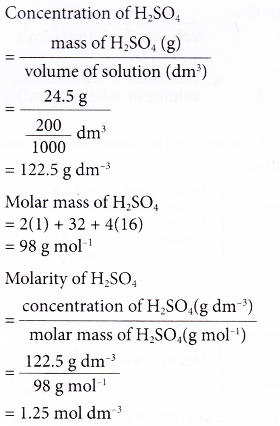
Concentration conversions using CASC - converting molarity to percent concentration, checking density

Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora

How to Calculate Analyte Concentration Using the Equivalence Point in an Acid-base Titration | Chemistry | Study.com