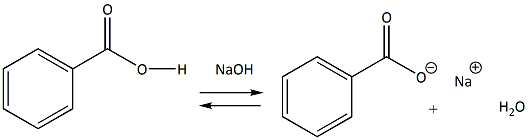
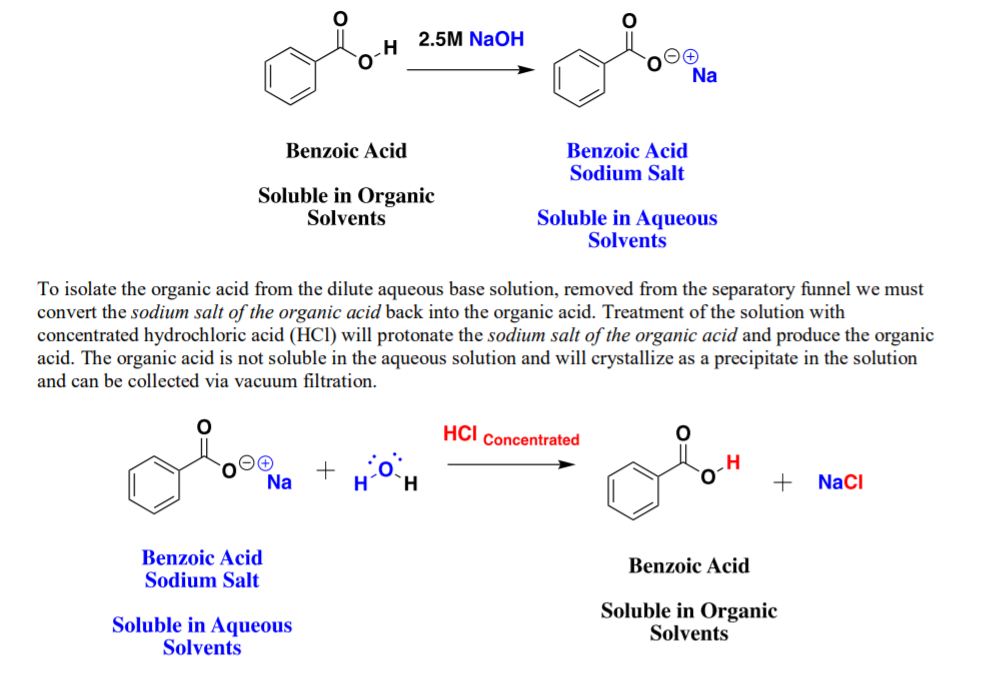
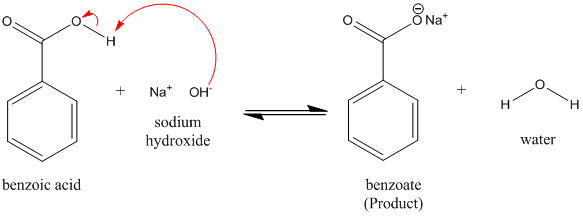
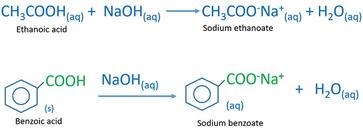
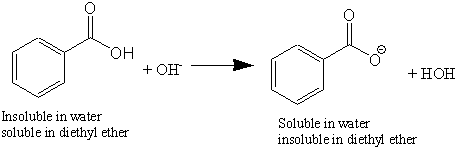
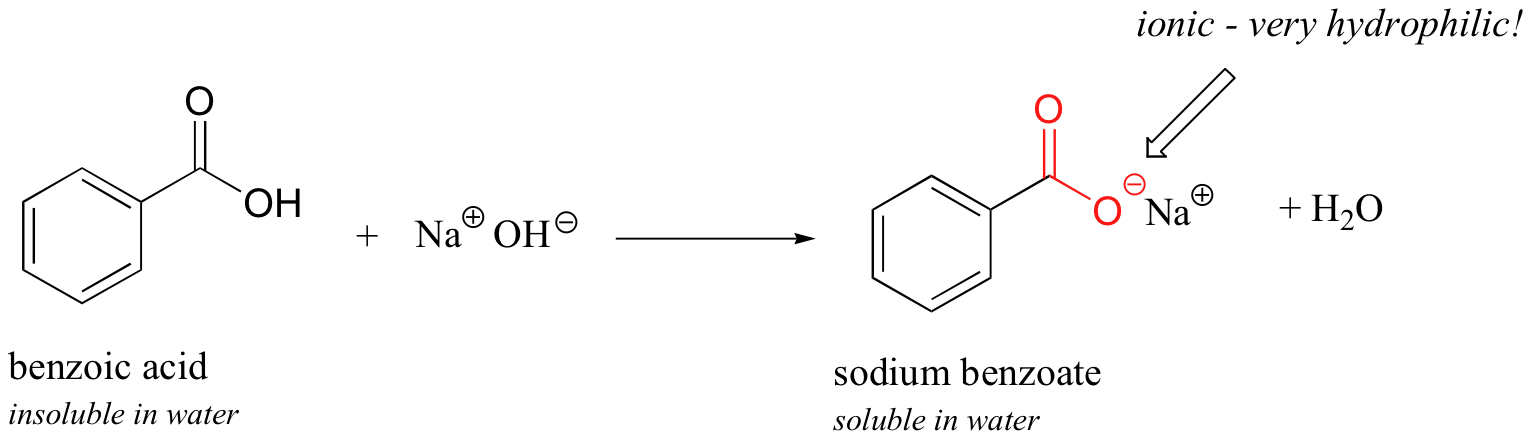
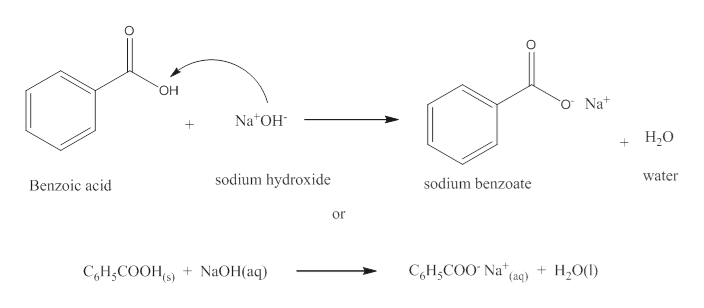
1. Draw a balanced chemical equation for the reaction that would occur between benzoic acid and aqueous sodium hydroxide. 2. Draw a balanced chemical equation for the reaction that would occur between
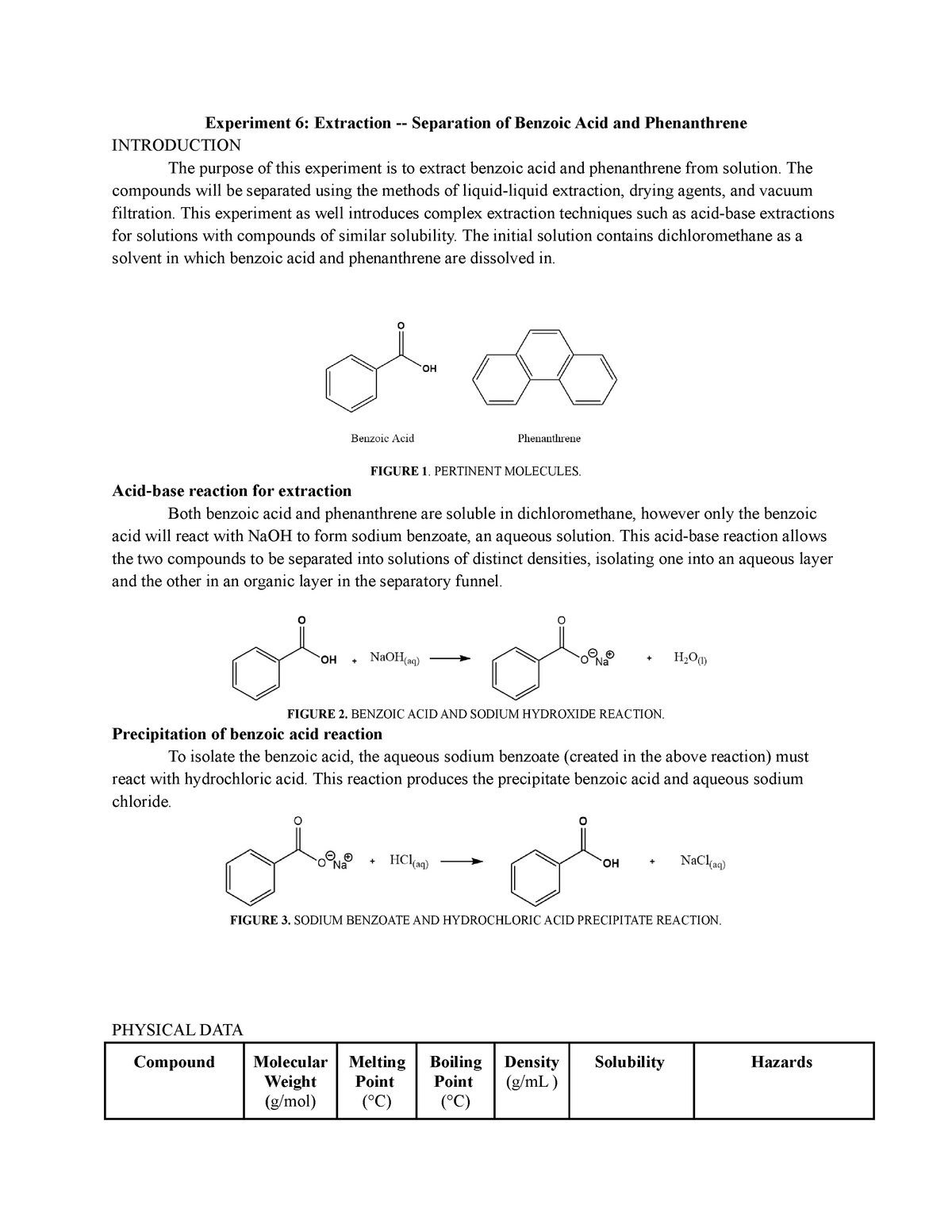
Experiment 6 Extraction - Separation of Benzoic Acid and Phenanthrene - Experiment 6: Extraction - - Studocu

Write a balanced equation for the reaction of benzoic acid with hydroxide ion. Why is it necessary to extract the ether layer with sodium hydroxide? | Homework.Study.com

OneClass: Benzoic acid is soluble in diethyl ether but not water, however, benzoic acid is extracted ...

Explain the results for the tube in which 1.0 m naoh was added to benzoic acid. write an equation for - Brainly.com
![When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] . When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .](https://dwes9vv9u0550.cloudfront.net/images/3890404/8b3b6f71-c797-41c4-8e11-5cf2fabbd29f.jpg)
When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .