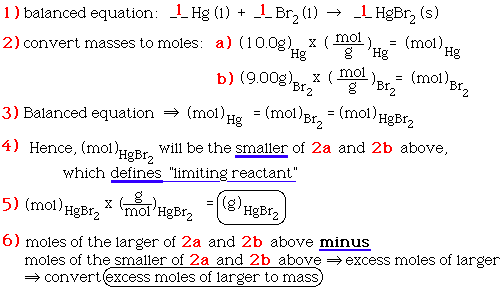![PDF] Dissolution kinetics of low grade complex copper ore in ammonia- ammonium chloride solution | Semantic Scholar PDF] Dissolution kinetics of low grade complex copper ore in ammonia- ammonium chloride solution | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/74104461ce1759e6c90926cb2fd02cdd1b4c9a35/2-Table1-1.png)
PDF] Dissolution kinetics of low grade complex copper ore in ammonia- ammonium chloride solution | Semantic Scholar
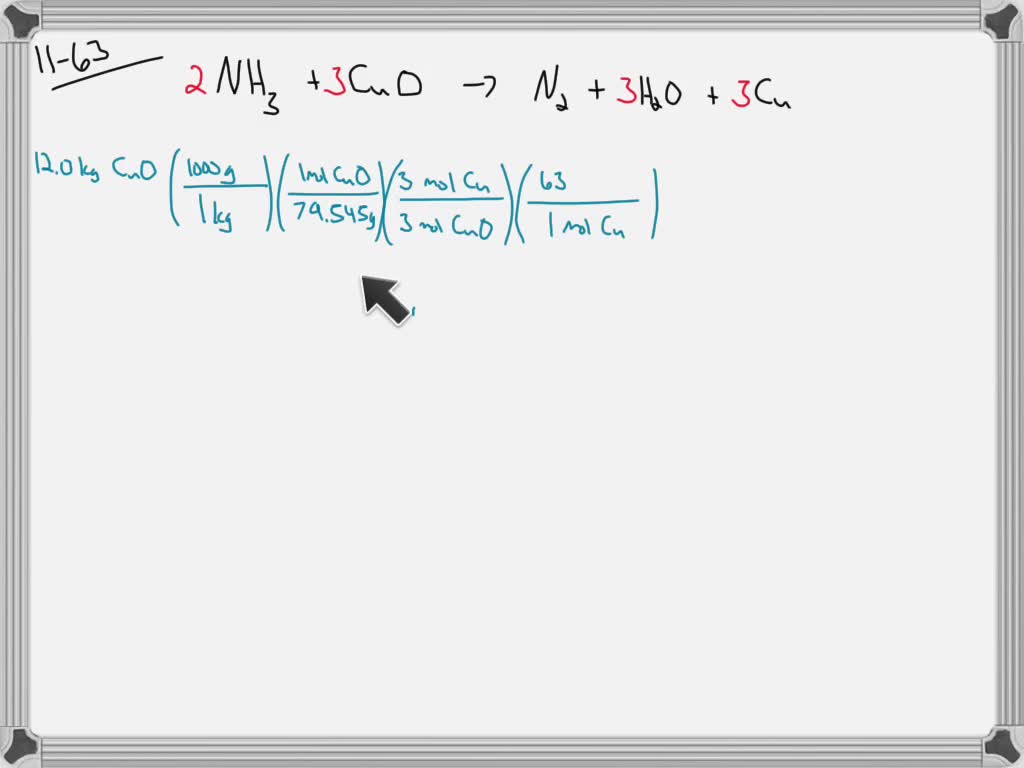
SOLVED: 1) Ammonia and copper (II) oxide react according to the following equation: 2NH3 + 3 CuO → N2 + 3 Cu + 3 H2O if 57.0 g of ammonia are combined
Facile in situ synthesis of copper nanoparticles supported on reduced graphene oxide for hydrolytic dehydrogenation of ammonia borane - RSC Advances (RSC Publishing)

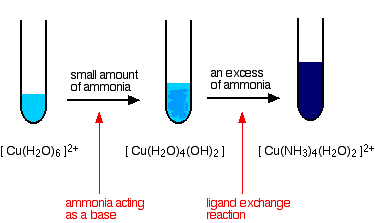
When dry ammonia gas is passed over hot copper (II) Oxide, a shinny brown residue and a colourless droplets are formed. Explain these two observations

Given that the relative molecular mass of copper oxide is 80 , what volume of ammonia measured at STP is required to completely reduce 120 g of copper oxide? The equation for

Give balanced equation for each of the following:Reduction of hot Copper (II) oxide to copper using ammonia gas:

When ammonia is passed over heated copper oxide, the metallic copper is obtained, the reaction shows - YouTube

Activating copper oxide for stable electrocatalytic ammonia oxidation reaction via in-situ introducing oxygen vacancies | SpringerLink
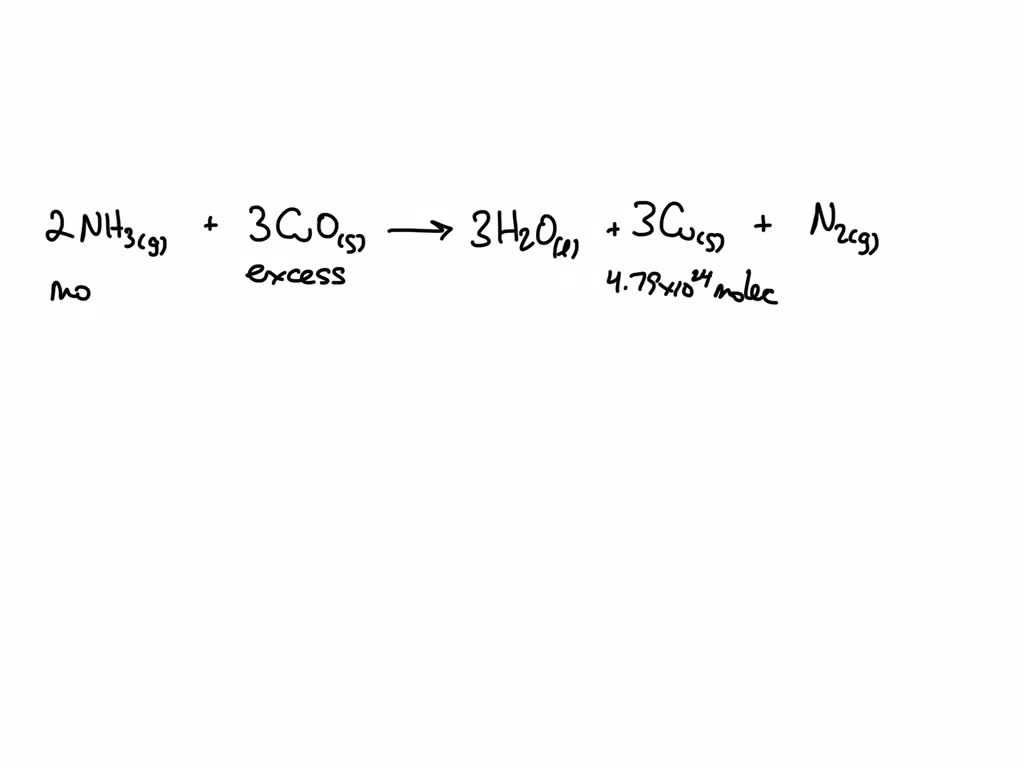
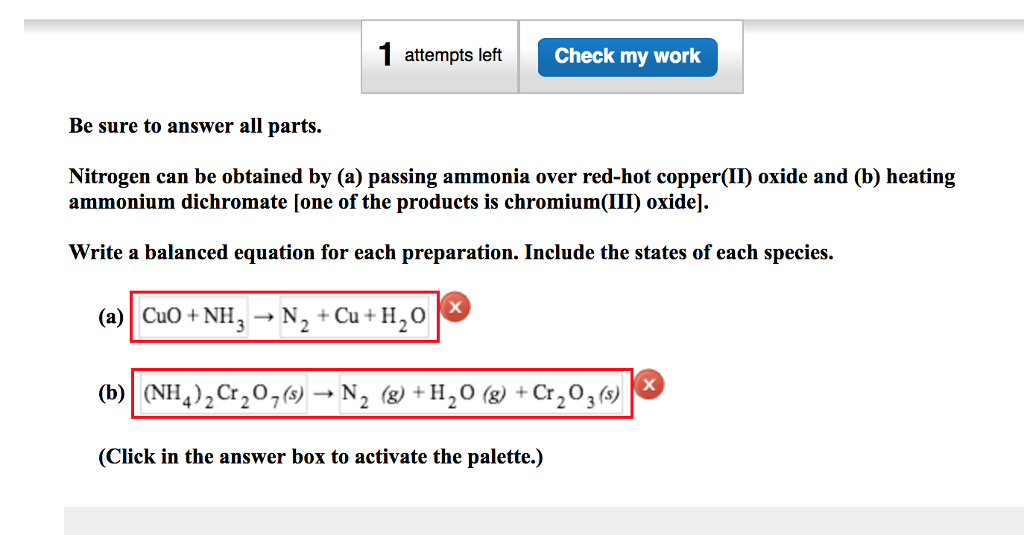
SOLVED: Ammonia gas and solid copper(II) oxide react to form liquid water, solid copper, and nitrogen gas. If copper(II) oxide is present in excess, how many moles of ammonia are needed to
![State one obervation for the following : Ammonia gas is passed over heated copper [II] oxide. | ... - YouTube State one obervation for the following : Ammonia gas is passed over heated copper [II] oxide. | ... - YouTube](https://i.ytimg.com/vi/7deWf3VEtQk/maxresdefault.jpg)
State one obervation for the following : Ammonia gas is passed over heated copper [II] oxide. | ... - YouTube
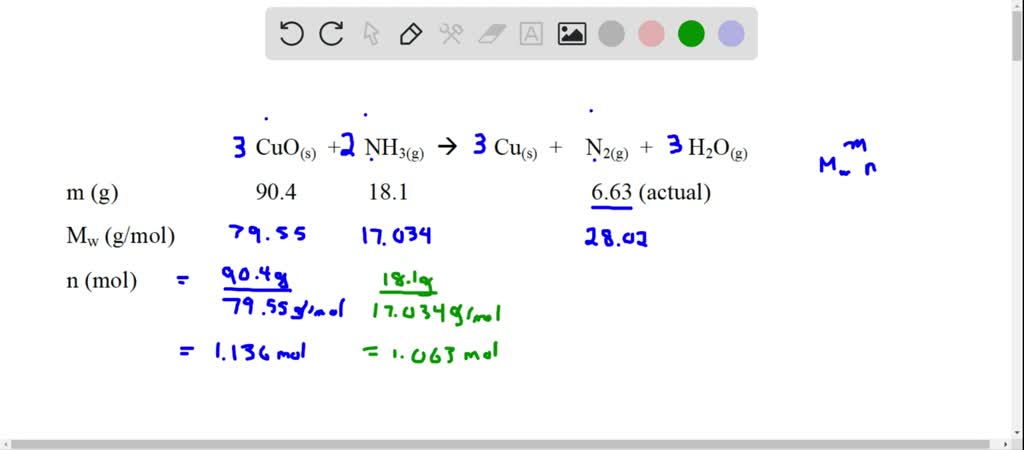
SOLVED: Gaseous ammonia, NH3, and copper(II) oxide, CuO, react together to form gaseous nitrogen (N2), solid copper, and water vapor. If a sample containing 17.9 g of NH3is reacted with 90.0 g
Dry ammonia gas was passed over heated lead (II) oxide and the products passed over anhydrous copper - Tutorke
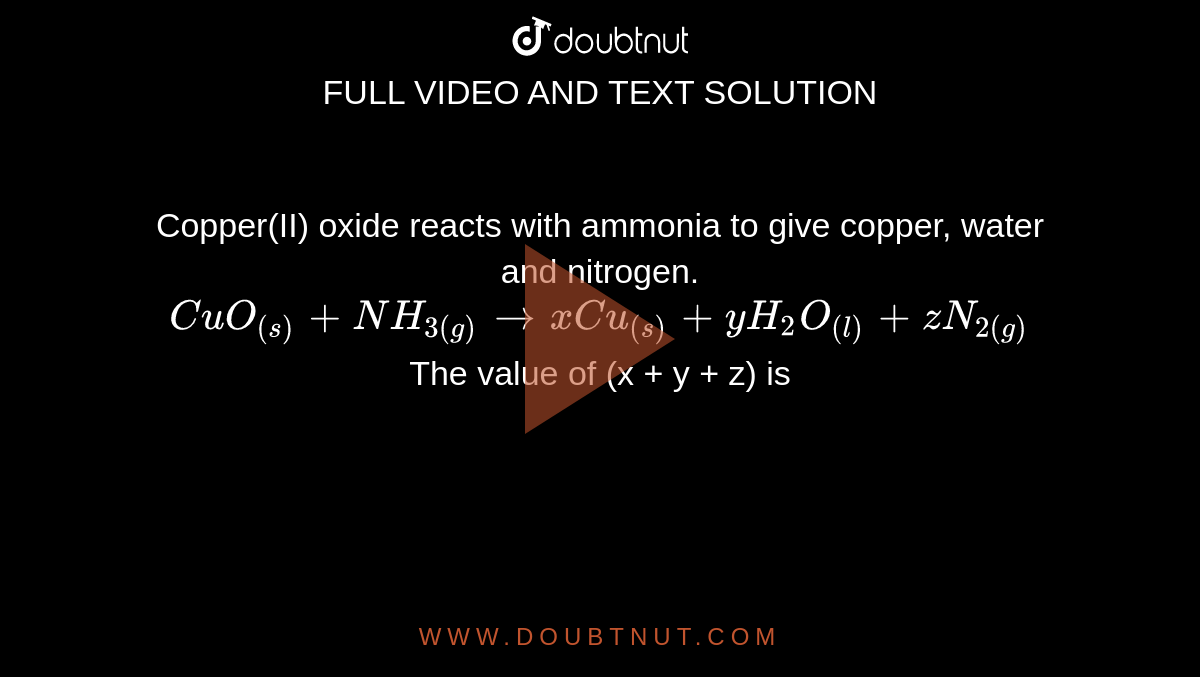
Copper(II) oxide reacts with ammonia to give copper, water and nitrogen. CuO((s)) +NH(3(g)) to xCu((s))+yH(2)O((l)) +zN(2(g)) The value of (x + y + z) is

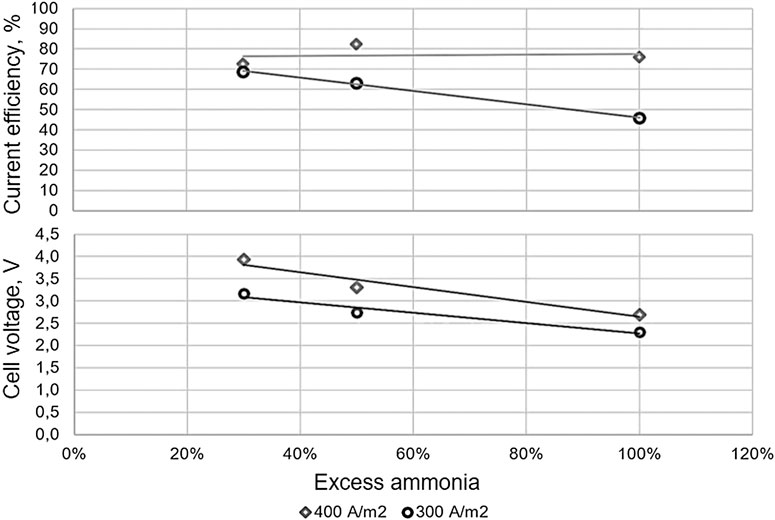
Coatings | Free Full-Text | Electro-Oxidation of Ammonia over Copper Oxide Impregnated γ-Al2O3 Nanocatalysts

Electrocatalytic, Homogeneous Ammonia Oxidation in Water to Nitrate and Nitrite with a Copper Complex | Journal of the American Chemical Society

When ammonia is passed over heated copper oxide, the metallic coper is obtained. The reaction shows - YouTube