Acetylene dibromide Molecular Weight - C2H2Br2 - Over 100 million chemical compounds | Mol-Instincts

Calculate the molar mass of the following substances (a) Ethyne C2H2 (b) Sulphur molecule S8 (c) Phophorous molecule P4 (d) Nitric acid HNO3 (e) Hydrochloride acid HCl .
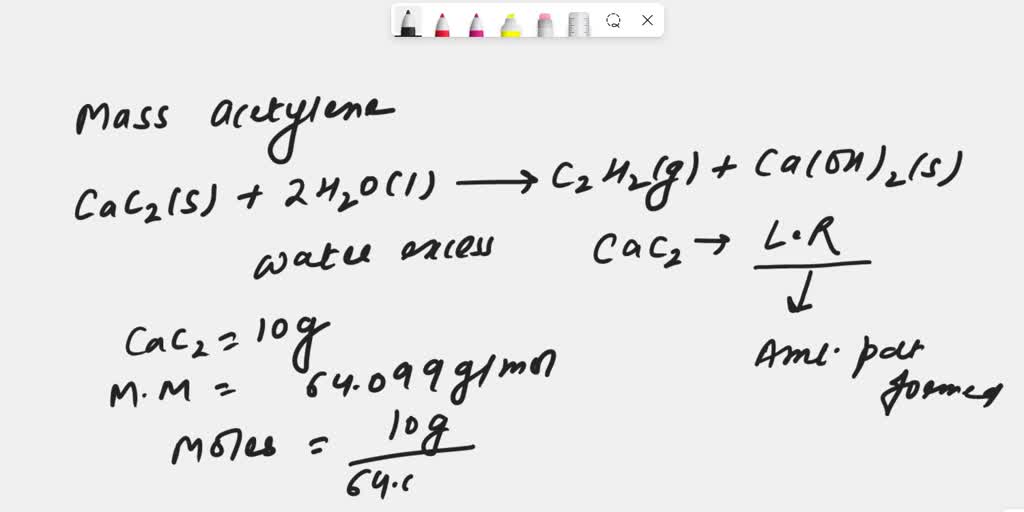
SOLVED: Calcium carbide is used in old-fashioned carbide lamps, in which water drips on carbide and the formed acetylene (C2H2) ignited: How many grams acetylene are produced when 10.0 g CaCz reacts

Calculate the molar mass of the following substances (a) Ethyne C2H2 (b) Sulphur molecule S8 (c) Phophorous molecule P4 (d) Nitric acid HNO3 (e) Hydrochloride acid HCl .









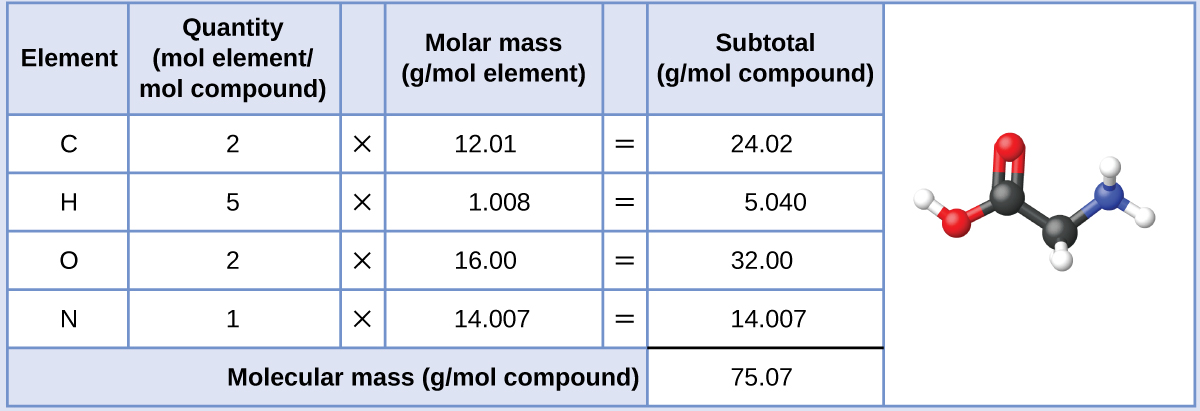

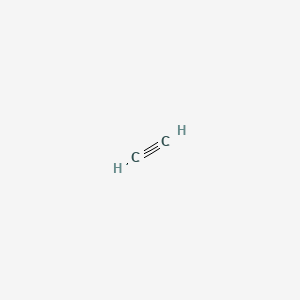
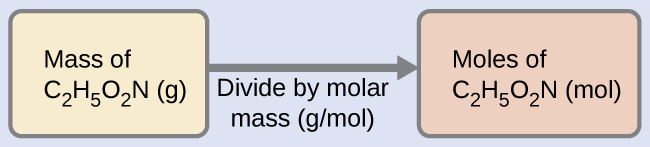
![Acetylene [C2H2] Molecular Weight Calculation - Laboratory Notes Acetylene [C2H2] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/11/acetylene-molecular-weight-calculation-300x192.jpg)