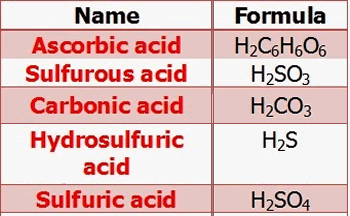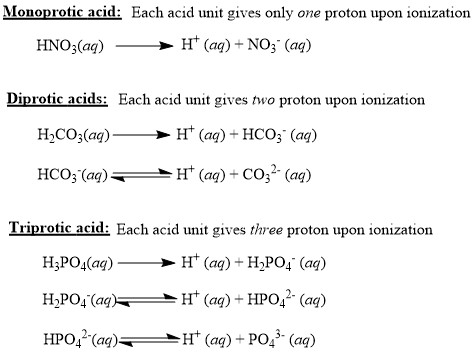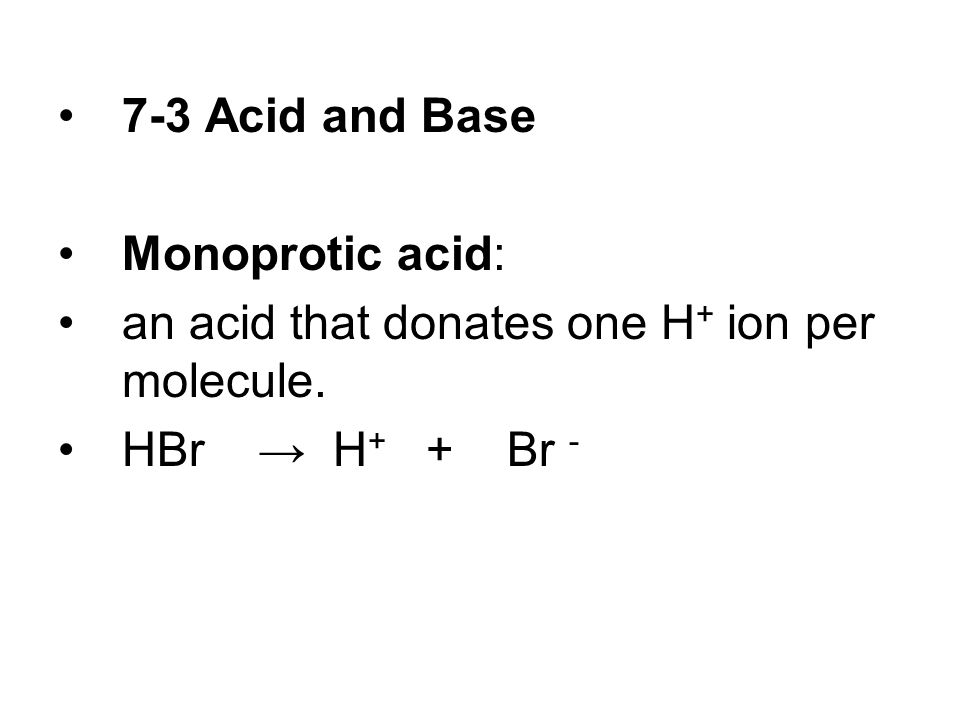
7-3 Acid and Base Monoprotic acid: an acid that donates one H + ion per molecule. HBr → H + + Br - - ppt download
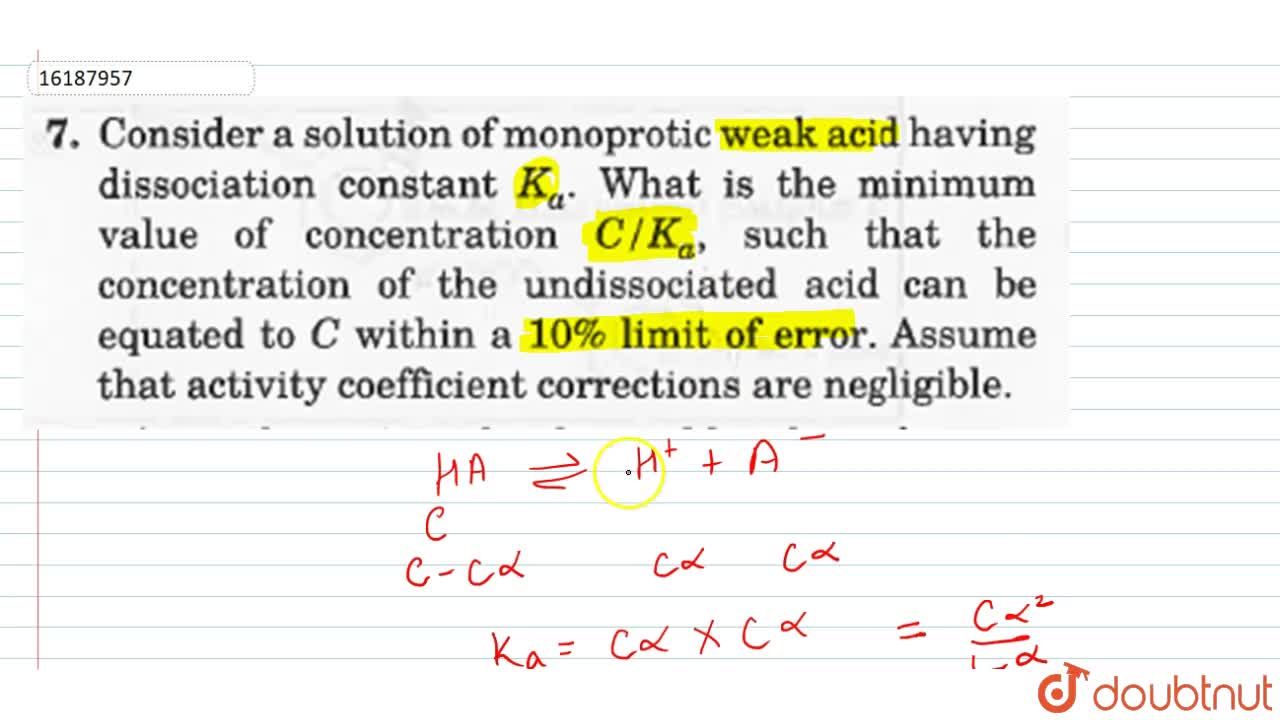
Consider a solution of monoprotic weak acid having dissociation constant K(a). What is the minimum value of concentration of the undissociated acid can be equated to C within a 10% limit of
19. Osmotic pressure of a mono protic acid in0.1 M aq. solution at temperature TK is 0.2 RT. pHof the solution will be(1 1(2) 2(3) 4(4) 3i thA colvent molecules move

Monoprotic Acid-Base Equilibria Review of Fundamentals 1.)Acids and Bases are essential to virtually every application of chemistry Analytical procedures. - ppt download
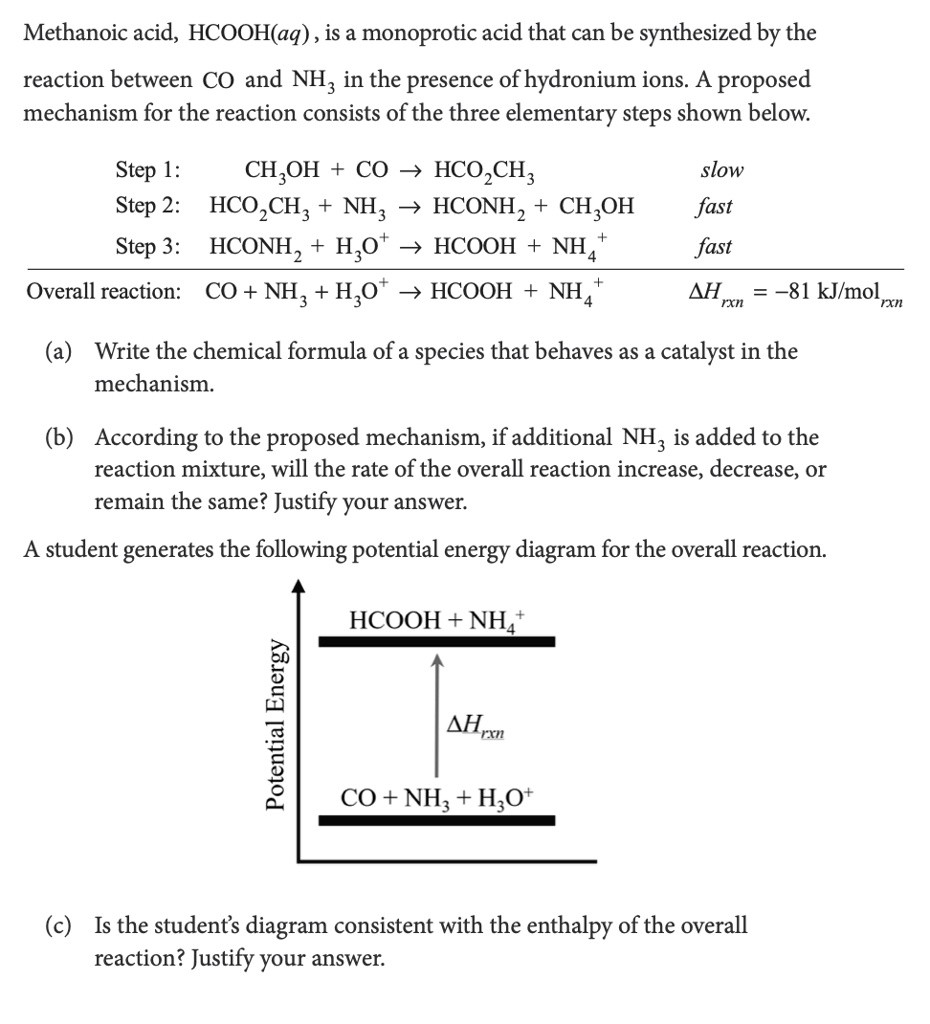
SOLVED: Methanoic acid, HCOOH(aq) is a monoprotic acid that can be synthesized by the reaction between CO and NH; in the presence ofhydronium ions proposed mechanism for the reaction consists of the